Laboratory of Cell Motility focuses on analysis of cell behavior and role of cytoskeleton in motility and proliferation of normal and tumor cells. We are interested in understanding, at a systems level, how specific features of cytoskeleton are coordinated with cell dynamics. Our studies using cultured normal and cancer mammalian cells are related to the development of anti-cancer therapy programs by in-depth analysis of cell behavior and proliferation in respect to different treatments.
We study different mitotic inhibitors, i.e. drugs stabilizing or depolymerizing microtubules, inhibitors of actin cytoskeleton and myosin and behavior of focal contacts – specific structures responsible for attachment of cell to the substrate and motility of cells on the solid substrates.
We implement high-throughput time lapse microscopy as well as different types of confocal microscopy analyzing living and fixed cells. Together with computer specialists we are developing image analysis tools for high-throughput analysis of cytoskeleton organization and cell motility. Other methods include but are not limited to flow cytometry (with imaging flow cytometry and spectral cytometry), knockdown technologies of different cytoskeletal components.
RESEARCH AREAS
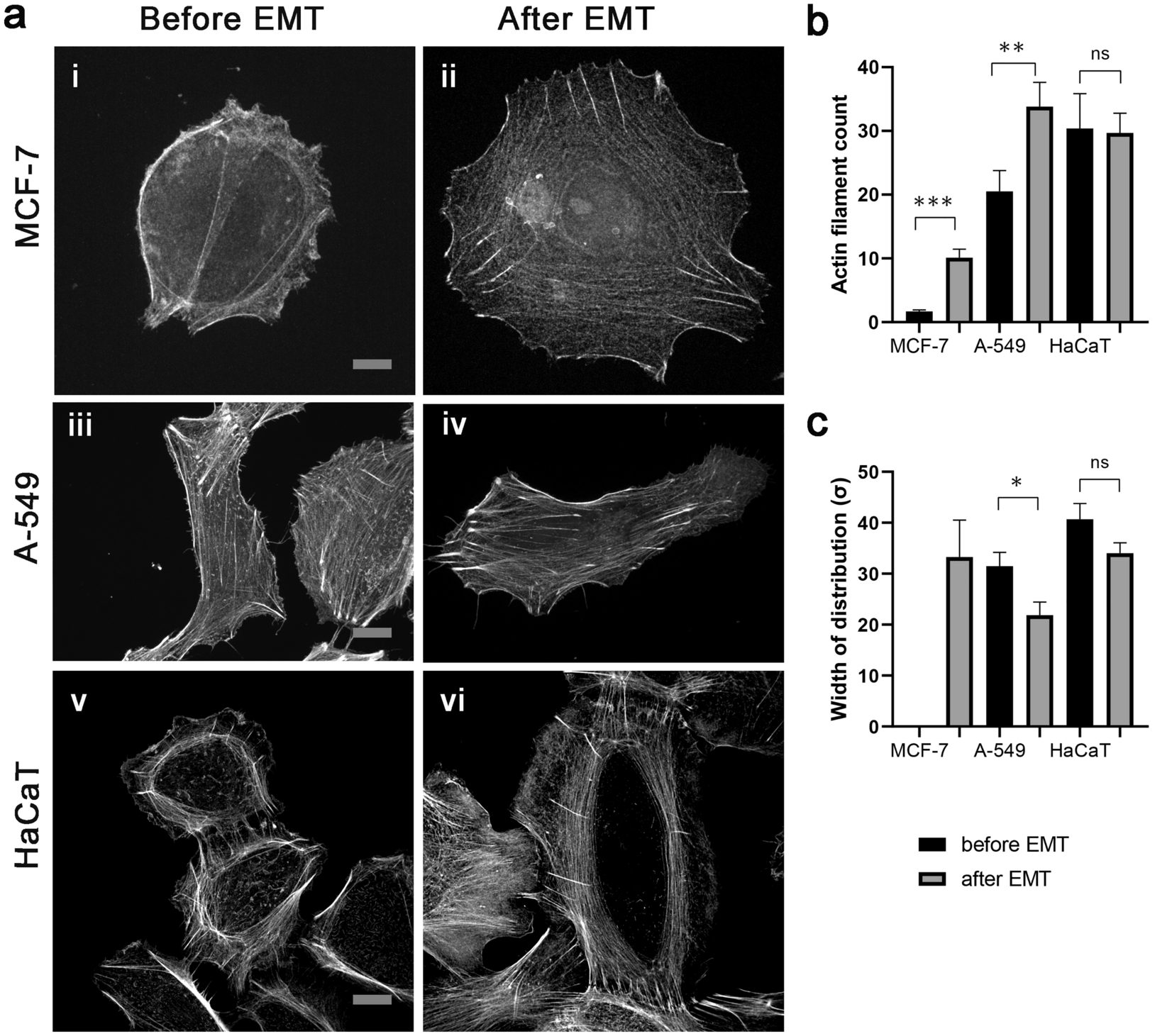
Actin filaments(shown in red) MCF-7 cells after EMT,where the GFP nucleus staining(shown in green)
We plan to assess the expression profile of actin and tubulin isoforms during induction of EMT in tumor cells and respective changes in the cytoskeleton behavior.
Find out more: Nurmagambetova, A., Mustyatsa, V., Saidova, A. et al. Morphological and cytoskeleton changes in cells after EMT. Sci Rep 13, 22164 (2023). https://doi.org/10.1038/s41598-023-48279-y
We evaluated FA dynamics by time-lapse microscopy with high spatial and temporal resolution, tracking FA size, lifetime, and intensity in A549 cells stably expressing Vinculin-RFP. We observed numerous small, short-living adhesions at the leading edge, occurring both at large lamellae aligning with the vector of cell body translocation and smaller lateral lamellae. That contrasted with the presence of only a few large adhesions at the trailing edge. Most FAs present at the trailing edge originated within lateral protrusions and only few of them formed directly there. During retraction of the rear cell edge, a portion of FAs has already been disassembled before retraction has reached them. However, some FAs at the trailing edge were enlarging, either through merging with neighboring FAs or gradual increase in area and. As a result, relatively large FAs were forcibly detached and rapidly disassembled only upon retraction edge reached them.
In the context of coordinated cell migration, discernible disparity between the dynamics of FAs emerges across different cell regions, where the lot of 'lilliputians' at the leading edge ultimately defeat the 'Gullivers' at the trailing edge.
The maturation of FAs continues through the involvement of focal adhesion kinase (FAK) and the mechanosensory proteins talin and vinculin, which provide formation of a mature FA structure and its association with actin microfilaments. FAK is a tyrosine kinase that becomes activated within FA, where it regulates cell adhesion, motility, and, probably, cell survival. FAK is one of the first specific proteins that is concentrated in FA and builds it through phosphorylation of specific proteins. FAK is often overexpressed in tumor cells, and can be a target for anticancer therapy. In the current project detailed studies of the role of FAK in the organization of FAs and cell motility will be performed.
In further studies we are going to test
hypothesis that FAK mediates the rapid formation and maturation of focal adhesion and associated cytoskeletal rearrangement playing central role in cell attachment, spreading, and motility.
Find out more: American Society for Cell Biology. 2023 ASCB Annual Meeting Abstracts. Mol Biol Cell. 2024 Jan 1;35(1):ar12. doi: 10.1091/mbc.E23-10-0402. PMID: 38108669; PMCID: PMC10881173.
- Histograms showing cell distribution of HeLa cells according to DNA content after 48 h treatment with anti-microtubule drugs. Minimal threshold concentrations (T1) for each drug are indicated by long arrow, and maximal threshold concentrations (T2)—by arrowhead.
- Time-lapse sequences illustrating three fates (mitotic slippage, death after mitotic slippage, and death in mitosis) exhibited by A549 cells under the treatment with 1 uM Taxol. Numbers indicate time from the moment of mitotic entry in hours. Mitochondrial membrane potential (red), active caspases 3/7 (green), and nuclei (blue) are indicated. Scale bar = 20 µm.
We currently are analyzing long-term consequences of mitotic arrest after washout of microtubule-stabilizing drugs. The initial event of relatively short treatment with microtubule stabilizers of cell cultures prone to mitotic slippage is formation of the numerous multi-nucleated cells. Restoration of normal divisions takes long time. We address the question what cells are the source of delayed recovery after drug washout.
Find out more: Suleimenov M, Bekbayev S, Ten M, Suleimenova N, Tlegenova M, Nurmagambetova A, Kauanova S and Vorobjev I (2022) Bcl-xL activity influences outcome of the mitotic arrest. Front. Pharmacol. 13:933112. doi: 10.3389/fphar.2022.933112
- Ivan VorobyevProfessor and Laboratory Head
- Marina JanibekovaJunior Researcher
- Arina Zhuravel
Research Assistant
- Vadim MustyatsaResearch Assistant
EQUIPMENT
● Long-term observation of physiological and morphological changes in normal and cancer cells
● Evaluate and document transfection rate and transfection stability
● Identification, quantification and qualification of fluorescently labeled cells, cell structures
● Multidimensional Acquisition: time lapse series, Z-stacks, multichannel, mosaic images, multiposition imaging, optical sectioning
Manufacturer Carl Zeiss
Model Cell Observer
Public Location C4, 607
Quantity 1
We have following light sources:
● Colibri LED illumination system: LED module 365 nm, LED module 455 nm, LED module 505 nm, LED module 590 nm
● HXP 120 illumination equipment (Metal-halide lamp as additional white light source)
We have following filtersets to observe samples: DAPI, HE eGFP, HE Cy 3, Cy 5, HE CFP+YFP, HE BFP + GFP + HcRed
